Ginkgo biloba (leaf)
Contents |
Nomenclature
Ginkgo biloba L. Ginkgoaceae
Standardized common name (English): ginkgo
Pinyin name(s): yin xing; bai guo (seed); yin xing ye (leaf)
Botanical Voucher Specimen
 |
 |
 |
|
|
|
|
|
|
Organoleptic Characteristics
|
Macroscopic Characteristics
|
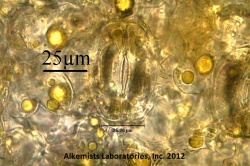
Microscopic Characteristics
 |
 |
|
|
|
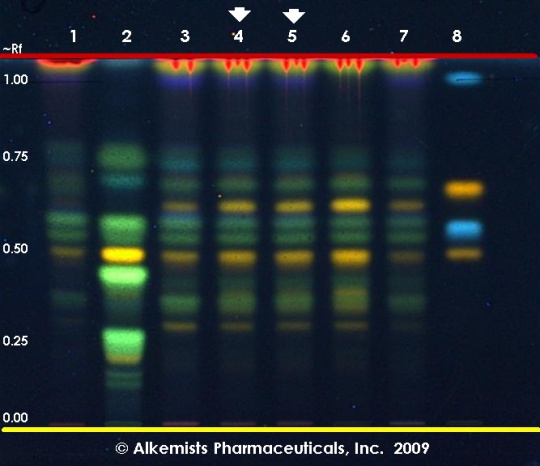
High Performance Thin Layer Chromatographic Identification
|
Ginkgo (leaf) Ginkgo biloba Lane Assignments Lane 1(3μl) Ginkgo biloba (leaf) (Vouchered Sample); Lane 2(3μl) Sophora japonica (fruit); Lane 3(3μl) Ginkgo biloba (leaf); Lane 4(3μl) Ginkgo biloba (leaf); Lane 5(3μl) Ginkgo biloba (leaf); Lane 6(3μl) Ginkgo biloba (leaf) (USA); Lane 7(3μl) Ginkgo biloba (leaf) (China) authenticated by macroscopic, microscopic &/or TLC studies according to the reference sources cited below held at Alkemists Pharmaceuticals, Costa Mesa, CA. Stationary Phase Silica gel 60, F254, 10 x 10 cm HPTLC plates Mobile Phase ethyl acetate: glacial acetic acid: formic acid: water [10/1.1/1.1/2.4] Sample Preparation Method 0.3 g + 3 ml 100% grain EtOH sonicated + heated @ 50° C ~ 1 hr. Detection Method Natural Product Reagent + PEG -> UV 365 nm Reference see British Pharmacopoeia, 2003
|
Supplementary Information
Ginkgo (leaf extract) (Ginkgo biloba) standardized to flavonol glycosides and terpenes.
General Characteristics AHPA recommends in its Known Adulterants list that appropriate steps be taken to assure that this raw material is free of the noted adulterant. Contact AHPA for additional information regarding relevant analytical methods or follow this link for more information.
Reported Adulterants Ginkgo (Ginkgo biloba) leaf extract with added flavonol glycosides or aglycones (e.g., rutin, quercetin, etc.).
Source: AHPA Known Adulterants [12]
Other Publications
HPLC identification, Ma, et al., 2016
An effective identification and quantification method for Ginkgo biloba flavonol glycosides with targeted evaluation of adulterated products,
Abstract. Ginkgo biloba L. (Ginkgoaceae) leaf extract is one of the most popular herbal products on the market, as it contains flavone glycosides (≥ 24%) and terpene lactones (≥ 6%), which are proposed to have significant physiological effects. Unfortunately, the challenging financial climate has resulted in a natural health product market containing adulterated ginkgo products. 42 ginkgo samples were analyzed to establish an HPLC profile for authentic ginkgo and common ginkgo adulterants, and to develop a method capable of easily detecting adulteration in ginkgo commercial products. In this study an efficient and targeted HPLC analysis method was established that is capable of distinguishing flavonol glycosides and aglycones simultaneously for the evaluation of ginkgo powdered extracts (PEs) and finished products in a single, 13 min run. Thirteen ginkgo leaf samples, fifteen standardized powdered extracts, and fourteen commercially available ginkgo products have been analyzed using this new HPLC method. Chromatograms were compared to six standard reference materials: one flavonol glycoside (rutin), three aglycones (quercetin, kaempferol and isorhamnetin), and two isoflavones (genestin and genistein). The quantitative chromatographic data was interpreted by principal component analysis (PCA), which assisted in the detection of unexpected chromatographic features in various adulterated botanical products. Only three of the commercially available ginkgo finished products tested in this study were determined to be authentic, with flavonol glycoside rutin, and aglycones quercetin, kaempferol, and isorhamnetin found to be common adulterants in the ginkgo powdered extract and finished product samples. Despite evidence of adulteration in most of the samples, each of the samples discussed herein met most of the current pharmacopeial standards. It is therefore critical that a preliminary evaluation be utilized to detect adulteration in commercial ginkgo products, prior to the acid hydrolysis procedure utilized in the current testing methods.[13]
UPLC/MS and HPTLC identification, Avula, et al., 2015
Identification of Ginkgo biloba supplements adulteration using high performance thin layer chromatography and ultra high performance liquid chromatography-diode array detector-quadrupole time of flight-mass spectrometry,
Abstract. Ginkgo biloba is one of the most widely sold herbal supplements and medicines in the world. Its popularity stems from having a positive effect on memory and the circulatory system in clinical studies. As ginkgo popularity increased, non-proprietary extracts were introduced claiming to have a similar phytochemical profile as the clinically tested extracts. The standardized commercial extracts of G. biloba leaf used in ginkgo supplements contain not less than 6% sesquiterpene lactones and 24% flavonol glycosides. While sesquiterpene lactones are unique constituents of ginkgo leaf, the flavonol glycosides are found in many other botanical extracts. Being a high value botanical, low quality ginkgo extracts may be subjected to adulteration with flavonoids to meet the requirement of 24% flavonol glycosides. Chemical analysis by ultra high performance liquid chromatography-mass spectrometry revealed that adulteration of ginkgo leaf extracts in many of these products is common, the naturally flavonol glycoside-rich extract being spiked with pure flavonoids or extracts made from another flavonoid-rich material, such as the fruit/flower of Japanese sophora (Styphnolobium japonicum), which also contains the isoflavone genistein. Recently, genistein has been proposed as an analytical marker for the detection of adulteration of ginkgo extracts with S. japonicum. This study confirms that botanically authenticated G. biloba leaf and extracts made therefrom do not contain genistein, and the presence of which even in trace amounts is suggestive of adulteration. In addition to the mass spectrometric approach, a high performance thin layer chromatography method was developed as a fast and economic method for chemical fingerprint analysis of ginkgo samples.[14]
Sources
- ↑ MOBOT, Tropicos.org http://www.tropicos.org/Image/35370
- ↑ Botanical Voucher Specimen Library, Alkemists Laboratories http://www.alkemist.com
- ↑ Botanical Voucher Specimen Library, Alkemists Laboratories http://www.alkemist.com
- ↑ Botanical Voucher Specimen Library, Alkemists Laboratories http://www.alkemist.com
- ↑ Steven Yeager, Mountain Rose Herbs http://www.MountainRoseHerbs.com
- ↑ Steven Yeager, Mountain Rose Herbs http://www.MountainRoseHerbs.com
- ↑ PlantaPhile http://plantaphile.com/
- ↑ PlantaPhile http://plantaphile.com/
- ↑ Elan M. Sudberg, Alkemist Laboratories http://www.alkemist.com
- ↑ Amy Brush, Traditional Medicinals http://www.traditionalmedicinals.com http://www.traditionalmedicinals.com
- ↑ Elan M. Sudberg, Alkemist Laboratories http://www.alkemist.com
- ↑ AHPA Known Adulterants http://www.ahpa.org/
- ↑ Ma, Y., Mani, A. Cai, Y., Thomson, J., Ma, J., Peudru, F., Chen, S., Luo, M., Zhang, J., Chapman, R., Shi, Z. 2016. An effective identification and quantification method for Ginkgo biloba flavonol glycosides with targeted evaluation of adulterated products Phytomedicine 23(4), 377-387. http://dx.doi.org/10.1016/j.phymed.2016.02.003
- ↑ Avula, B., Sagi, S., Gafner, S., Upton, R., Wang, Y.H., Wang, M., Khan, I.A. 2015. Identification of Ginkgo biloba supplements adulteration using high performance thin layer chromatography and ultra high performance liquid chromatography-diode array detector-quadrupole time of flight-mass spectrometry Analytical and Bioanalytical Chemistry 407(25), 7733-7746. http://dx.doi.org/10.1007/s00216-015-8938-1
- Botanical
- Ginkgoaceae
- Media
- Voucher
- MOBOT, Tropicos.org
- Botanical Voucher Specimen Library, Alkemists Laboratories
- Organolepsy
- Steven Yeager, Mountain Rose Herbs
- Macroscopy
- PlantaPhile
- Microscopy
- Elan M. Sudberg, Alkemist Laboratories
- Amy Brush, Traditional Medicinals http://www.traditionalmedicinals.com
- HPTLC
- AHPA Known Adulterants