Vaccinium myrtillus (fruit)
Nomenclature
Vaccinium myrtillus L. Ericaceae
Standardized common name (English): bilberry
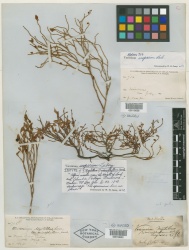
Botanical Voucher Specimen
|
|
|
Organoleptic Characteristics
Macroscopic Characteristics
|
|
|
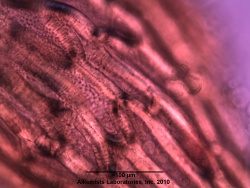
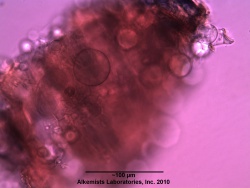
Microscopic Characteristics
|
High Performance Thin Layer Chromatographic Identification
|
Bilberry (fruit) (Vaccinium myrtillus) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
Reference Sample(s) Reference: Dissolve 2 mg of pelargonin in 5 mL of methanol. Dissolve 2 mg of delphinidin in 5 mL of methanol. Optional: dissolve 2 mg of malvidin in 5 mL of methanol. Stationary Phase Stationary phase, i.e. Silica gel 60, F254 Mobile Phase 1-Butanol, formic acid, water 65:16:19 (v/v/v) Sample Preparation Method Sample: Mix 1 g of powdered sample with 10 mL of methanol and sonicate for 10 minutes, then centrifuge or filter the solutions and use the supernatants / filtrates as test solutions. Derivatization reagent: no derivatization Detection Method Saturated chamber; developing distance 70 mm from lower edge; relative humidity 33% Other Notes Images presented in this entry are examples and are not intended to be used as basis for setting specifications for quality control purposes. System suitability test: Pelargonin: red to orange zone at Rf ~ 0.89; Delphinidin: violet zone at Rf ~ 0.80 Identification: Compare result with reference images. The fingerprint of the test solution is similar to that of the corresponding botanical reference sample. Additional weak zones may be present. The chromatogram of the test solution shows four violet to violet-blue zones between Rf ~ 0.46 and 0.58. Test for adulteration: No intense violet zone is seen at Rf ~ 0.35 (Mallow flower). There is neither a blue zone at Rf ~ 0.37 nor a violet zone at Rf ~ 0.44 (Roselle flower).
|
Supplementary Information
ABC Botanical Adulterants Prevention Program Laboratory Guidance
Bilberry (fruit extract) (Vaccinium myrtillus)
General Characteristics This ABC BAPP Laboratory Guidance Document presents an overview and comparison of various analytical technologies and methods used to differentiate between authentic bilberry extracts and non-authentic materials, with particular discussion of known chemical markers of other anthocyanin-rich fruit extracts, synthetic dyes, and a detailed literature review (including HPLC and HPTLC characterization methods).
Source: ABC, Botanical Adulterants Prevention Program [9]
AHPA Known Adulterants List
Bilberry (fruit extract) (Vaccinium myrtillus)
General Characteristics AHPA recommends in its Known Adulterants list that appropriate steps be taken to assure that this raw material is free of the noted adulterant. Contact AHPA for additional information regarding relevant analytical methods or follow this link for more information.
Reported Adulterants Red dye #2 (amaranth dye)
Source: AHPA Known Adulterants [10]
AHPA Practical: Detecting Artificial Dye Adulteration in "Bilberry" Extracts
Introduction
The American Herbal Products Association is providing here analytical tools and methods to identify adulteration of powdered raw materials labeled as bilberry (Vaccinium myrtillus) extract. There are well-established scientifically valid methods for determining the presence of red dye in materials purported to be powdered bilberry extracts. They are provided now as immediately available practical tools for industry in order to deal appropriately with reports of adulteration of this ingredient and make wise purchasing decisions.
"Bilberry" Extracts Adulterated with Artificial Dyes
In the process of evaluating commercial material labeled as bilberry extract, AHPA member company MediHerb investigated a sample that appeared to meet the listed specification for anthocyanin content by simple spectrometry. However upon further analysis it was discovered that this material was not likely derived from bilberry and was in fact adulterated with amaranth dye (red dye no. 2). Their work has since been published (see: Bilberry adulteration using the food dye amaranth. Penman KG, Halstead CW, Matthias A, De Voss JJ, Stuthe JM, Bone KM, Lehmann RP. J Agric Food Chem. 2006 Sep 20;54(19):7378-82).
Subsequent to the work by Penman et al., Steven Dentali, PhD, AHPA’s vice president of scientific and technical affairs, authored an article published in the trade journal Nutraceuticals World, which can be accessed here. This article warns that lower price material available from nontraditional sources can be an initial indication of an adulterated extract. This is especially problematic where common methods of analysis are easily “cheated” so that a specification is met, but the material is not what it should be.
The information presented here is intended to educate industry about the potential quality problem with ingredients labeled as bilberry extract and to provide a scientifically valid method to aid in the differentiation between genuine bilberry fruit extract and material adulterated with amaranth dye. When examined with these methods by qualified individuals, information should be obtained that will allow more informed purchasing decisions than may be available with single wavelength spectroscopic analysis.
Methods for Detection of Dye in "Bilberry" Extracts
Anthocyanins turn blue at elevated pH
Bilberry extracts are blue-black in color but form a pink solution when diluted at neutral pH. At elevated pH, anthocyanins change color and anthocyanin-containing solutions, such as can be made by diluting bilberry extract with water, turn blue. This is illustrated here where the far left solution is very dilute true bilberry extract, next is this solution with the pH adjusted to greater than 10, next is dilute fake bilberry extract under the same conditions, and lastly very dilute fake bilberry extract.
This experiment is simple to replicate with bilberry extract or any other anthocyanin-containing materials and so is not necessarily indicative of bilberry anthocyanins. In fact this principle is easily observable by adding baking soda to dilute anthocyanin containing solutions. A “kitchen test” using diluted red wine, or grape juice, or other suitable (anthocyanin-containing) juice can be performed to illustrate this property of anthocyanins. Sprinkling baking soda into such a prepared dilute reddish solution will cause it to turn purple or bluish. This same color transformation will happen with bilberry (or other anthocyanin containing) extract, but does not occur with amaranth dye.
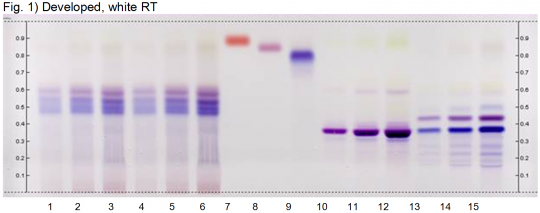
High performance thin layer chromatography (HPTLC)
Authentic bilberry can be easily differentiated from materials adulterated with amaranth dye under the HPTLC conditions used to create the image shown here. Lanes 1 and 4 are authentic bilberry extract. The horizontal colored bands represent anthocyanin compounds. The adulterated samples in lanes 2 and 3 have a different pattern of colored bands and, most notably, a bright red band that corresponds to amaranth dye. The last lane shows what pure amaranth dye looks under these same conditions.
Click here for the HPTLC conditions used to produce this result.
Other Relevant Sources of Bilberry Information
AHP and USP monographs
The American Herbal Pharmacopoeia and Therapeutic Compendium has published a bilberry fruit monograph that includes standards of analysis, quality control, and therapeutic information. More information can be found at [1].
The United States Pharmacopeia has published a dietary supplement monograph for powdered bilberry extract as an in-process revision in the Pharmacopeial Forum, July–Aug. 2007;33(4):685-688.
Other compositional analysis and quantification of bilberry anthocyanins
The Journal of Agricultural and Food Chemistry has published a method of anthocyanin analysis in bilberry. That abstract can be accessed here.
A more recent paper published by the Journal of AOAC INTERNATIONAL “was developed and validated for the identification and quantification of both anthocyanins and anthocyanidins present in bilberry extracts and products.” The abstract for it is available here.
NUTRAfoods published a review of ten commercial bilberry extracts using USP/NF Pharmacopeial methods of analysis for anthocyanin content. Deficiences were noted in labeling and content of many products. That article can be accessed here.
Acknowledgements
AHPA appreciates the contributions of member companies MediHerb for the technical work and CAMAG Scientific Inc. for the HPTLC method.
Sources
- ↑ Images courtesy of the C.V. Starr Virtual Herbarium of the New York Botanical Garden http://sciweb.nybg.org/science2/VirtualHerbarium.asp
- ↑ Images courtesy of the C.V. Starr Virtual Herbarium of the New York Botanical Garden http://sciweb.nybg.org/science2/VirtualHerbarium.asp
- ↑ PlantaPhile http://plantaphile.com/
- ↑ PlantaPhile http://plantaphile.com/
- ↑ Elan M. Sudberg, Alkemist Laboratories http://www.alkemist.com
- ↑ Elan M. Sudberg, Alkemist Laboratories http://www.alkemist.com
- ↑ Elan M. Sudberg, Alkemist Laboratories http://www.alkemist.com
- ↑ HPTLC Association http://www.hptlc-association.org/
- ↑ ABC, Botanical Adulterants Prevention Program http://cms.herbalgram.org/BAP/LGD/BilberryLabGuidanceDocument.html
- ↑ AHPA Known Adulterants http://www.ahpa.org/