Foeniculum vulgare (fruit)
(add askbox) |
(add PlantaPhile references) |
||
| Line 73: | Line 73: | ||
principal ridges and six large vittae. | principal ridges and six large vittae. | ||
}} | }} | ||
| + | {{ Media2 | cat=Macroscopy | ||
| + | | source=PlantaPhile | ||
| + | | mainimage=PlantaPhile - 862.jpg | ||
| + | | companyimage=PlantaPhile logo.jpg | ||
| + | | companyURL=http://plantaphile.com/ | ||
| + | |||
| + | | source2=PlantaPhile | ||
| + | | image2=PlantaPhile - 2588.jpg | ||
| + | | companyimage2=PlantaPhile logo.jpg | ||
| + | | companyURL2=http://plantaphile.com/ | ||
| + | | }} | ||
|} | |} | ||
=Microscopic Characteristics= | =Microscopic Characteristics= | ||
Latest revision as of 21:15, 5 May 2015
Contents |
Nomenclature
Foeniculum vulgare Mill. Apiaceae
Syn. mishreya; shatapushpa
Standardized common name (English): fennel
Pinyin name(s): hui xiang; xiao hui xiang (fruit)
Botanical Voucher Specimen
 |
|
|
|
|
Organoleptic Characteristics
|
Macroscopic Characteristics
|
Microscopic Characteristics
|
High Performance Thin Layer Chromatographic Identification
|
Sweet Fennel (fruit) (Foeniculum vulgare ssp. vulgare var. dulce) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
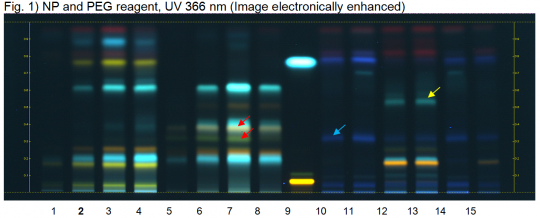
Reference Sample(s) Reference: Dissolve 3 mg of rutin in 1 mL of methanol. Dissolve 1 mg of caffeic acid in 1 mL of methanol. Stationary Phase Stationary phase, i.e. Silica gel 60, F254 Mobile Phase Ethyl acetate, formic acid, water 15:1:1 (v/v/v) Sample Preparation Method Sample: Mix 500 mg of powdered sample with 5 mL of methanol and sonicate for 10 minutes, then centrifuge or filter the solutions and use the supernatants / filtrates as test solutions. Derivatization reagent: NP and PEG reagent, Preparation NP reagent: 1 g of natural products reagent in 200 ml ethyl acetate, Preparation PEG reagent: 10 g of polyethylene glycol 400 in 200 mL dichloromethane, Use: Heat the plate at 100°C for 5 min, dip (time 0, speed 5) while still hot, dry in air. Detection Method Saturated chamber; developing distance 70 mm from lower edge; relative humidity 33% Other Notes Images presented in this entry are examples and are not intended to be used as basis for setting specifications for quality control purposes. System suitability test: Rutin: orange fluorescent zone at Rf ~ 0.07; Caffeic acid: light bluish fluorescent zone at Rf ~ 0.77. Identification: Compare result with reference images. The fingerprint of the test solution is similar to that of the corresponding botanical reference sample. Additional weak zones may be present. The chromatogram of the test solution shows a yellow zone at Rf ~ 0.17, a light blue zone at Rf ~ 0.20, a faint yellow zone at Rf ~ 0.26 and a faint blue zone at Rf ~ 0.32. A light blue zone is seen in the center part of the chromatogram at Rf ~ 0.53 (yellow arrow). Test for other species: No intense dark blue zone is seen at Rf ~ 0.78 (white arrow, Bitter Fennel fruit). No light blue zone is seen at Rf ~ 0.62 (red arrow, Anise fruit or Caraway fruit). No brownish zones are seen at Rf ~ 0.32 and 0.38 (green arrows, Caraway fruit).
|
|
Fennel (seed) (Foeniculum vulgare) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
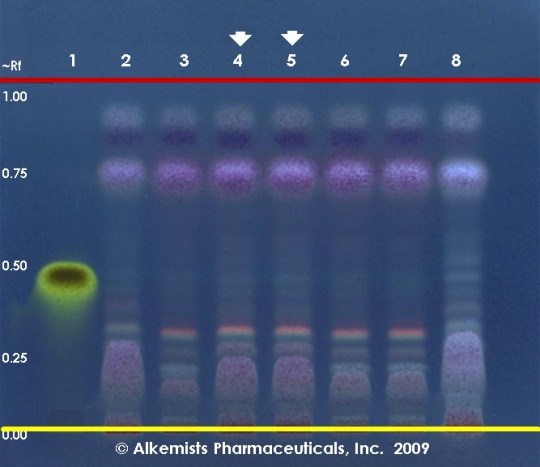
Reference materials used here have been authenticated by macroscopic, microscopic &/or TLC studies according to the reference source cited below held at Alkemists Laboratories, Costa Mesa, CA. Stationary Phase Silica gel 60, F254, 10 x 10 cm HPTLC plates Mobile Phase toluene: ethyl acetate [9.5/0.5] Sample Preparation Method 0.5g+5ml dichloromethane, sonicate/centrifuge/decant, evaporate to dryness with N2, qs 1.0 ml Toluene Detection Method Vanillin/H2SO4 Reagent -> 110° C 5 min -> UV 365 nm Reference see British Pharmacopoeia, 2003
|
|
Anise fruit (Pimpinella anisum) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
Reference Sample(s) Reference: Dissolve 3 mg of rutin in 1 mL of methanol. Dissolve 1 mg of caffeic acid in 1 mL of methanol. Stationary Phase Stationary phase, i.e. Silica gel 60, F254 Mobile Phase Ethyl acetate, formic acid, water 15:1:1 (v/v/v) Sample Preparation Method Sample: Mix 500 mg of powdered sample with 5 mL of methanol and sonicate for 10 minutes, then centrifuge or filter the solutions and use the supernatants / filtrates as test solutions. Derivatization reagent: NP and PEG reagent; Preparation NP reagent: 1 g of natural products reagent in 200 mL ethyl acetate; Preparation PEG reagent: 10 g of polyethyleneglycole 400 in 200 mL dichloromethane; Use: Heat for 3 min to 100°C, dip (time 0, speed 5) in NP reagent while still hot, dry, dip in PEG reagent. Detection Method Saturated chamber; developing distance 70 mm from lower edge; relative humidity 33% Other Notes Images presented in this entry are examples and are not intended to be used as basis for setting specifications for quality control purposes. System suitability test: Rutin: orange fluorescent zone at Rf ~ 0.07; Caffeic acid: light bluish fluorescent zone at Rf ~ 0.77. Identification: Compare result with reference images. The fingerprint of the test solution is similar to that of the corresponding botanical reference sample. Additional weak zones may be present. In the upper part of the chromatogram there are three prominent zones: a light blue zone at Rf ~ 0.88, a yellow zone at Rf ~ 0.76 and a light blue zone at Rf ~ 0.61. In the lower part of the chromatogram there is a sequence of three zones (yellow, light blue, yellowish) between Rf ~ 0.15 and 0.26. Right above the application position there is a yellow zone. Test for adulteration: In the middle of the chromatogram there are neither yellow zones at Rf ~ 0.32 and Rf ~ 0.38 (red arrows, Caraway fruit) nor a faint dark blue zone at Rf ~ 0.32 (blue arrow, Bitter Fennel fruit) nor a light blue zone at Rf ~ 0.53 (yellow arrow, Sweet Fennel fruit).
|
|
Bitter Fennel (fruit) (Foeniculum vulgare ssp. vulgare var. vulgare) Lane Assignments Lanes, from left to right (Track, Volume, Sample):
Reference Sample(s) Reference: Dissolve 3 mg of rutin in 1 mL of methanol. Dissolve 1 mg of caffeic acid in 1 mL of methanol. Stationary Phase Stationary phase, i.e. Silica gel 60, F254 Mobile Phase Ethyl acetate, formic acid, water 15:1:1 (v/v/v) Sample Preparation Method Sample: Mix 500 mg of powdered sample with 5 mL of methanol and sonicate for 10 minutes, then centrifuge or filter the solutions and use the supernatants / filtrates as test solutions. Derivatization reagent: NP and PEG reagent; Preparation NP reagent: 1 g of natural products reagent in 200 mL ethyl acetate; Preparation PEG reagent: 10 g of polyethylene glycol 400 in 200 mL dichloromethane; Use: Heat the plate at 100°C for 5 min, dip (time 0, speed 5) in NP reagent while still hot, dry in air, dip in PEG reagent. Detection Method Saturated chamber; developing distance 70 mm from lower edge; relative humidity 33% Other Notes Images presented in this entry are examples and are not intended to be used as basis for setting specifications for quality control purposes. System suitability test: Rutin: orange zone at Rf ~ 0.07; Caffeic acid: light bluish zone at Rf ~ 0.77. Identification: Compare result with reference images. The fingerprint of the test solution is similar to that of the corresponding botanical reference sample. Additional weak zones may be present. The chromatogram of the test solution shows a dark blue zone at Rf ~ 0.78 slightly above the position of reference caffeic acid and a dark blue zone at Rf ~ 0.31 (white arrows). Test for other species: No yellow zone is seen at Rf ~ 0.17 (red arrows, Anise fruit, Sweet Fennel fruit). A light blue zone is neither seen at Rf ~ 0.53 (yellow arrow, Sweet Fennel fruit) nor at Rf ~ 0.62 (yellow arrow, Anise fruit, Caraway fruit). No brownish zones are seen at Rf ~ 0.32 and 0.38 (green arrows, Caraway fruit).
|
Supplementary Information
Sources
- ↑ MOBOT, Tropicos.org http://www.tropicos.org/Image/100013734
- ↑ Trish Flaster, MSc, Botanical Liaisons, LLC http://www.BotanicalLiaisons.com
- ↑ United States Dispensatory (1918)
- ↑ United States Dispensatory (1918)
- ↑ PlantaPhile http://plantaphile.com/
- ↑ PlantaPhile http://plantaphile.com/
- ↑ United States Dispensatory (1918)
- ↑ Elan M. Sudberg, Alkemist Laboratories http://www.alkemist.com
- ↑ Elan M. Sudberg, Alkemist Laboratories http://www.alkemist.com
- ↑ HPTLC Association http://www.hptlc-association.org/
- ↑ Elan M. Sudberg, Alkemist Laboratories http://www.alkemist.com
- ↑ HPTLC Association http://www.hptlc-association.org/
- ↑ HPTLC Association http://www.hptlc-association.org/